Recently updated on January 13th, 2026 at 02:58 pm
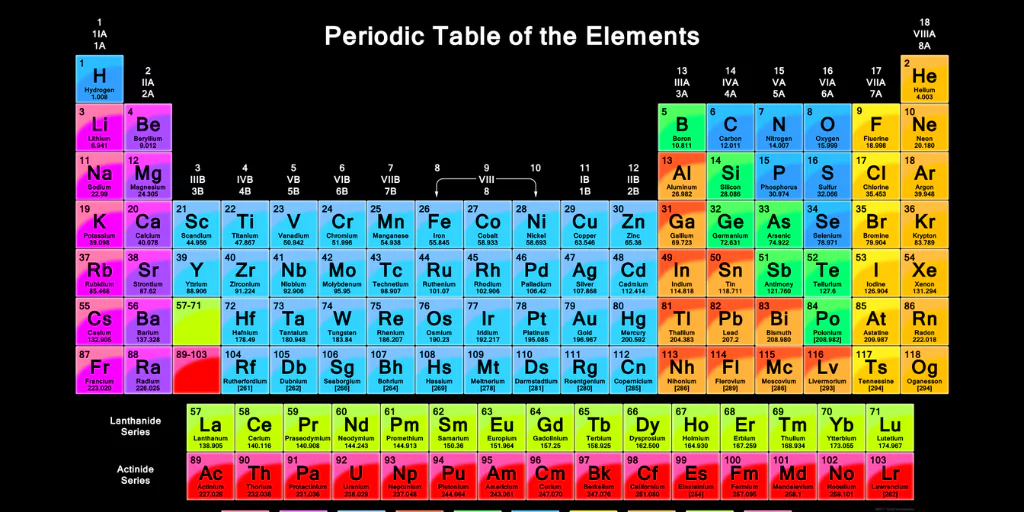
The Periodic Table is one of science’s most well-known and useful tools. Often referred to as the “map of elements,” it arranges all known chemical elements in a fashion that emphasises their features, similarities, and interactions. From classroom textbooks to advanced research facilities, the periodic table is the foundation of chemistry and a key to understanding the universe‘s building blocks.
What is a Periodic Table?
The periodic table is a tabular arrangement of chemical elements ordered mostly by atomic number (the number of protons in an atom’s nucleus). Each element is identified by its chemical symbol, atomic number, and relative atomic mass.
Its structure enables scientists to swiftly discover not only an element’s properties, but also how it may interact with others.
History of the Periodic Table

- Dmitri Mendeleev (1869): Known as the “father of the periodic table,” Mendeleev first arranged the elements by their atomic masses and noticed repeating patterns in their properties.
- Modern Table (20th century): Later, the table was refined by arranging elements by atomic number (Henry Moseley’s discovery). This corrected earlier anomalies and created the modern structure we use today.
Structure of the Periodic Table
The table is divided into rows (periods) and columns (groups or families):
Periods (Rows): Horizontal rows that show elements with increasing atomic numbers. As you move across a period, properties like atomic radius, ionisation energy, and electronegativity gradually change.
Groups (Columns): Vertical columns where elements share similar chemical and physical properties. For example:
- Group 1: Alkali metals (highly reactive, soft metals)
- Group 17: Halogens (reactive non-metals)
- Group 18: Noble gases (inert, stable gases)
Also see: Work, Energy and Power – Difference Explained Simply
Major Element Categories
1. Metals – Found on the left and centre of the table; good conductors of heat and electricity (e.g., iron, copper, aluminium).
2. Non-metals – Located on the right side; poor conductors, often gases or brittle solids (e.g., oxygen, nitrogen, sulfur).
3. Metalloids – Elements with properties of both metals and non-metals (e.g., boron, silicon).
Importance of the Periodic Table
- Predicting Properties: By knowing an element’s group and period, scientists can predict how it reacts.
- Chemical Bonding: Helps in understanding ionic and covalent bonding patterns.
- Discovering New Elements: The periodic table provides placeholders for elements yet to be discovered.
- Everyday Applications: From medicines to materials like plastics, metals, and fuels—the periodic table connects science to daily life.
Fun Facts
- There are currently 118 confirmed elements.
- Some elements are named after famous scientists (e.g., curium after Marie Curie, einsteinium after Albert Einstein).
- Noble gases like helium and neon glow brightly when electrified, used in balloons and neon lights.
Conclusion
The periodic table is more than just a chart; it serves as a global language of chemistry. It aids in understanding the structure, properties, and behaviour of matter, making it a vital tool for students, scientists, and companies worldwide. As science improves, the periodic table evolves, and new elements may be discovered in the future.