Every living organism depends on thousands of chemical reactions to survive—breaking down food, building proteins, generating energy, and repairing cells. Normally, these responses would be too sluggish to sustain life. Enzymes come into play in this situation—biological catalysts known as enzymes quicken chemical reactions within cells without consuming themselves.
1. What Are Enzymes?
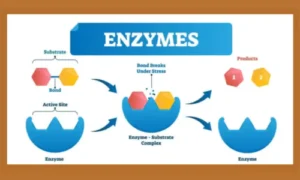
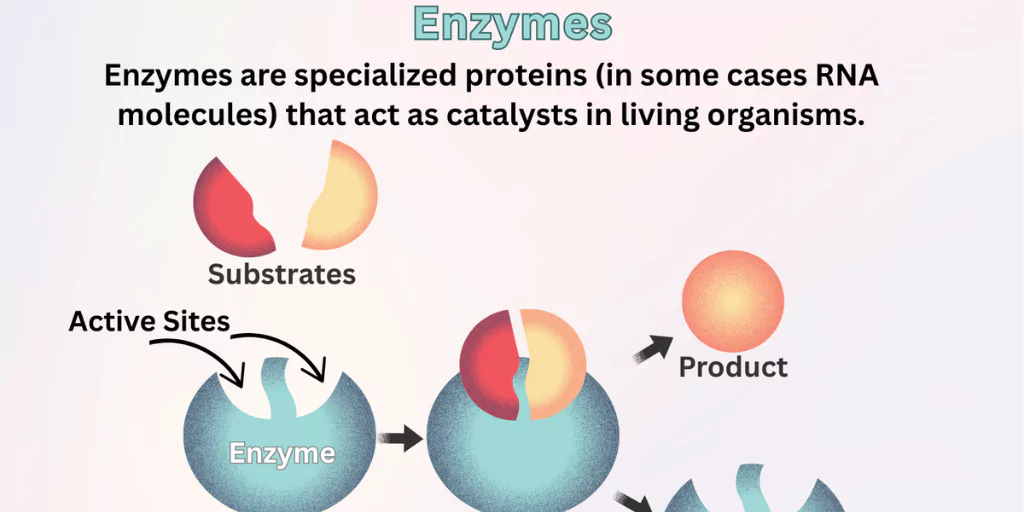
Enzymes are proteins (and in some cases RNA molecules) that increase the rate of biochemical reactions. Without enzymes, life’s essential processes, such as digestion, energy production, and DNA replication, would be far too slow to sustain life.
2. Structure of Enzymes
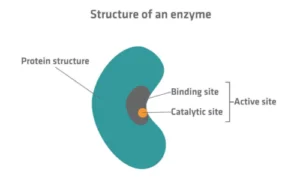
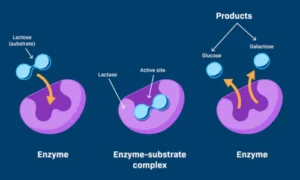
- Active Site: The specific region where the substrate (reactant) binds.
- Enzyme-Substrate Complex: Temporary union of enzyme and substrate.
- Product Formation: After the reaction, products are released, and the enzyme is free to catalyse another reaction.
The “lock and key” model and the “induced fit” model explain how enzymes bind to substrates with precision.
3. How Enzymes Work
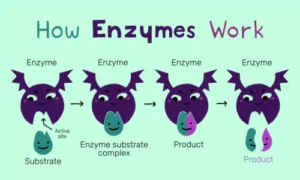
Reactions happen more quickly when enzymes reduce the activation energy, or the energy required to initiate a process.
Steps of enzyme action:
1. Substrate binds to the enzyme’s active site.
2. An enzyme catalyses the reaction by stabilising the transition state.
3. The product is formed and released.
4. Enzyme remains unchanged and ready for reuse.
4. Types of Enzymes
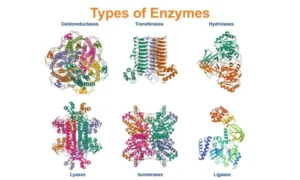
Enzymes are classified based on the reactions they catalyse:
1. Oxidoreductases – Involved in oxidation-reduction (e.g., dehydrogenase).
2. Transferases – Transfer functional groups (e.g., kinase).
3. Hydrolases – Break bonds with water (e.g., amylase, lipase).
4. Lyases – Add or remove groups without hydrolysis.
5. Isomerases – Rearrange molecules (e.g., mutase).
6. Ligases – Join molecules together (e.g., DNA ligase).
5. Factors Affecting Enzyme Activity
- Temperature: Too high or too low temperatures can denature enzymes.
- pH: Each enzyme has an optimum pH (e.g., pepsin works best in acidic conditions).
- Substrate concentration: Higher concentration increases activity until saturation.
- Enzyme inhibitors: Molecules that block enzyme activity (competitive or non-competitive).
6. Importance of Enzymes in Cells
- Metabolism: Controls the breakdown of glucose (cellular respiration).
- Digestion: Enzymes like amylase, protease, and lipase help break down food.
- DNA Replication & Repair: DNA polymerase and ligase ensure genetic stability.
- Detoxification: Liver enzymes break down toxins.
- Signal Regulation: Enzymes help transmit cellular signals for growth and repair.
7. Industrial & Medical Applications
1. Food sector: Enzymes include rennin for cheese making and amylase for beer brewing.
2. Medicine: Enzymes as drugs (streptokinase dissolves blood clots).
3. Biotechnology: DNA-modifying enzymes in genetic engineering.
4. Diagnostics: Enzymes used in glucose tests and pregnancy kits.
Conclusion
Enzymes are the molecular motors that drive life. They reduce activation energy, making cellular activities more efficient and sustainable. Enzymes play a crucial role in life, from digestion to DNA repair. Their applications in medicine, industry, and biotechnology demonstrate their importance beyond the cell in influencing modern research and healthcare.






66b
August 23, 202566b cung cấp đa dạng phương thức thanh toán phù hợp với thói quen người dùng Việt Nam. Quá trình nạp tiền được thực hiện tức thì, trong khi rút tiền thường hoàn tất trong vòng 15 phút đến 24 giờ tùy phương thức. TONY12-082
66b
August 23, 202566b cung cấp đa dạng phương thức thanh toán phù hợp với thói quen người dùng Việt Nam. Quá trình nạp tiền được thực hiện tức thì, trong khi rút tiền thường hoàn tất trong vòng 15 phút đến 24 giờ tùy phương thức. TONY12-082
888slot apk
August 23, 2025Nhà cái ghi điểm ở việc ứng dụng AI và Big Data để phân tích hành vi người chơi, 888slot apk từ đó tối ưu hóa giao diện và tính năng theo thói quen của từng thị trường. TONY12-082
ab-resurs
August 23, 2025Тяговые аккумуляторные https://ab-resurs.ru батареи для складской техники: погрузчики, ричтраки, электротележки, штабелеры. Новые АКБ с гарантией, помощь в подборе, совместимость с популярными моделями, доставка и сервисное сопровождение.